Which of the Following Indicates an Endothermic Reaction
However endothermic reactions do occur spontaneously or naturally. In an endothermic reaction energy is absorbed during the reaction and the products thus have a larger quantity of energy than the reactants.
Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal.

. Note that the final total volume is 100 mL in each case due to the addition of H 2 O. 3 Types of Chemical Reactions Notes Synthesis - two or more elements or compounds combine to form one compound. Chemical Reaction Engineering 3rd Edition by Octave Levenspiel.
Negative enthalpy change for a reaction indicates exothermic process while positive enthalpy change corresponds to endothermic process. The degree of randomness of the system the symbol Δ indicates the change in the variable that occurs when the reactants are transformed into the productsHydrogen bonds are exothermic 3 and the heat derives from. At this time the zeolite catalyst is used for an effective endothermic reaction.
Energy change binding energy of products - binding energy of reactants -394 2 x -242 -. As always all work must be shown to receive full credit. In this work the effect of metal foam catalyst on the compn.
10th in white bookcase by Keyes 310. Of the product was investigated by using zeolite with a metal foam support as a catalyst for the endothermic. Double Replacement -.
Calculate the concentrations of NCS- and Fe3 in each of the following solutions. The endothermic reaction of the fuel is used as a cooling system for hypersonic aircraft. I-lipase ii-trypsin iii-pepsin.
The pH scale shows how acidic a substance is. Therefore the reaction would not occur without some outside influence such as persistent heating. Identify gas A in the following experiment.
The energy contained in the chemical bonds of reactants and products and entropy S ie. Identify the option that indicates the correct enzyme that is secreted in location A B and C. Free energy change has two components.
States that if an overall reaction takes place in several steps its standard reaction enthalpy is the sum of the standard enthalpies of the intermediate reactions at the same. Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactionsIn enzyme kinetics the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzymes kinetics in this way can reveal the catalytic mechanism of this enzyme its role in metabolism how its activity is controlled and how a drug or a modifier.
We can also calculate the energy change when this reaction takes place. Decomposition reaction involves. Due before 9 AM Fri Feb.
The reaction mixture became hot. Decomposition of vegetable matter into compost is an endothermic reaction. Learn what pH means and how it is measured in this KS3 chemistry guide from BBC Bitesize.
Decomposition-a single compound decomposes into two or more elements or smaller compounds.
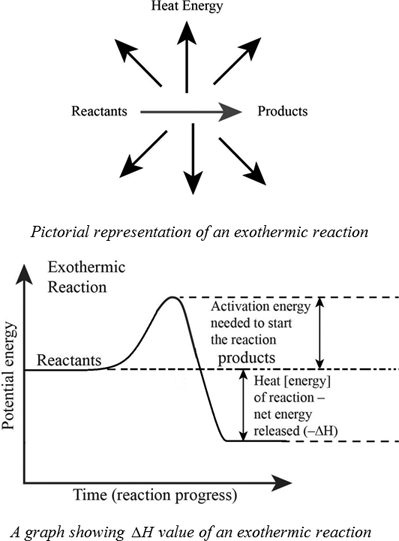
Definition Of Exothermic And Endothermic Reactions Chegg Com

Endothermic Vs Exothermic Reactions Chemtalk

Endothermic Reaction Definition Equation Graph Examples

Schematic Representation Of The Energy Level Diagram Of An Exothermic Download Scientific Diagram

The Picture Above Is Showing An Example Of Endothermic Because It S Taking Energy From The Things Around It W Chemistry Physical Chemistry Exothermic Reaction

Endothermic Or Exothermic Reaction Of The Vo 2 Phase Caused By Phase Download Scientific Diagram

H Is A State Function Because E P V Are State Functions So It Depends Only On The Difference Betwee Chemistry Education Teaching Chemistry Science Chemistry

Reactions In Which Energy Is Released Are Exothermic Reactions And Those That Take In Heat Energy Are Exothermic Reaction Chemistry Lessons Teaching Chemistry

Endothermic Vs Exothermic Reaction Graphs Youtube
Are Endothermic Reactions Faster Than Exothermic Quora

Endothermic Exothermic Reactions Energy Changes In Chemical Reactions Mcat Content

Enthalpy Vs Entropy What Is Delta H And Delta S Video Lesson Transcript Study Com
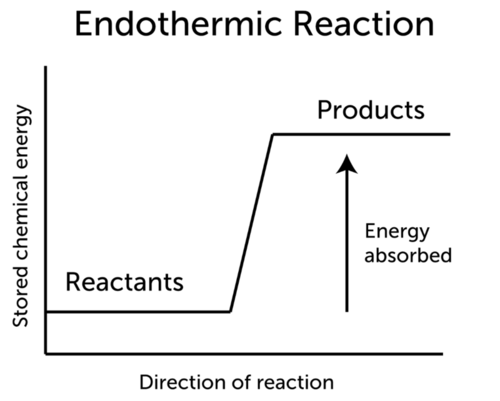
Endothermic Reaction Ck 12 Foundation
In An Endothermic Reaction Is All Of The Activation Energy Absorbed If Not Can An Endothermic Reaction Release Any Heat If So Why Isn T It Exothermic Quora
In An Endothermic Reaction The Energy Of Products Is More Than The Reactants Means They Have More Heat I Have Assumed More Energy To Be More Heat Energy So Why Does The

Heat Of Reaction Reflects The Difference In Enthalpy Between The Products And The Reactants Teaching Chemistry Chemistry Education Teaching Science

Endothermic Lettering System Energy



Comments
Post a Comment